Jump to:
Purpose
This test differentiates immune cell sub-populations via flow cytometry.
Description: increased CD4-positive T cell number (MP:0008074), decreased CD4-positive T cell number (MP:0008075), etc., ...Experimental Design
- Minimum number: 3M + 3F
- Age at test: Week 16
- Sex: Both (sexually dimorphic)
Equipment
- Scissors and forceps for biopsy
- Precision balance
- Calibrated single and multichannel pipettes
- Plate shaker
- Refrigerated centrifuge
- Flow Cytometer (capable of distinguishing a minimum of 8 colours per well)
- Tissue dissociator:
- GentleMACS tissue dissociator OR
- Equipment for manual dissociation
- Cell counter equipment:
- Orflo Moxi-Z Cell counter OR
- Coulter Vicell XR OR Life Technologies Attune® Flow Cytometer OR
- Haemocytometer
- 96-well V-bottomed plates (Falcon #353263)
- Petri dishes
- Dispensing troughs
- Low retention pipette tips for antibody solutions
- (if using GentleMACS for dissociation) C Tubes. It is acceptable to re-use these once.
- 50ml Falcon tubes
- Cell strainers e.g. 70μm cell strainers that fit 50ml Falcon tubes (BD Falcon, #352350) OR 70-80µM Nytex
- Cell counter recipients (i.e., slides/cassettes/etc. for cell counter)
- (if sample processing delayed) RPMI 1640
- (if sample processing on same day) HBSS, with phenol red
- CS (calf serum)
- PBS with Mg2+, with Ca2+ (for enzyme buffer used for DNAse and Collagenase D digestions)
- PBS without Mg2+, without Ca2+ (for FACS buffer to be used in all steps subsequent to enzymatic digest)
- EDTA (0.5M stock; final concentration 2mM)
- Digestion enzyme (Collagenase D from Roche #11088858001), stock solution in enzyme buffer (see below), aliquoted and stored at –20°C
- DNAse I stock solution (Sigma, #DN25) in enzyme buffer (see below), aliquoted and stored at -20°C
- RBC lysis buffer (eBioscience #00-4300-54 or BD Biosciences #555899, both 10X from manufacturer)
- HEPES (pH 7.2-7.4)
Procedure
This protocol requires several steps in the collection, preparation and analysis of the samples. Each one is detailed separately below.
Reagent preparation
Note that two different PBS solutions are required for the protocol below, one with Ca2+ and Mg2+, another without Ca2+ and Mg2+.
- Collection buffer:
- (if spleens are to be processed on the same day) HBSS with Ca2+ and Mg2+ and phenol red (e.g. Life Technologies 14170161) OR
- (if analysis will be delayed) RPMI medium with 2% CS added.
- FACS buffer (for all steps subsequent to enzymatic digest; stable for up to 1 month in the fridge):
- PBS 1X without Ca2+/Mg2+ OR
- HBSS 1X without Ca2+/Mg2+
- EDTA 2mM
- 2% (v/v) CS
- 10mM HEPES, pH 7.2-7.4
- Brilliant Stain Buffer (BD 563794; for all steps when two or more brilliant violet antibodies are used to prevent non-specific dye-to-dye interaction)
- Enzyme buffer (for DNAse and Collagenase D digestions; Stable for up to 1 month in the fridge):
- PBS with Ca2+ and Mg2+ OR
- HBSS 1X with Ca2+/Mg2+
- 2% (v/v) CS;
- 10mM HEPES, pH 7.2-7.4
- RBC Lysis buffer: Prepare a 1X solution in ddH20 from 10X stock lysis buffer.
- Stopping buffer (require 300 µl per sample):
- 1x PBS without Ca2+ and Mg2+ or 1X HBSS without Ca2+ and Mg2+
- 0.1 M EDTA (37.5 g/L)
- Antibody cocktails for Panels 1 & 2
- Protect antibodies and prepared cocktails from direct light.
- Final concentration of antibodies should be determined by titration to ensure saturating amounts of antibody are used. Appropriate amounts of antibodies can be mixed together from the manufacturer’s stock solutions and stored for 1 week at 4ºC prior to dilution in FACS buffer immediately before use. Do NOT pre-mix BV antibodies. These should be added fresh to the diluted staining mixure.
- Each sample will require 50 µl (or up to 100 µl) of diluted 1X antibody cocktail.
- Antibody cocktails should be gently but thoroughly mixed to ensure homogeneity of the solutions.
- In order to eliminate aggregated antibodies from your mix, centrifuge each antibody cocktail for 8 min at 20,000xg and 8°C prior to staining cells.
- Antibody Panels
- Recommended antibody (marker) panels, Panel A for T, NKT and NK cells, Panel B for B, myeloid and NK cells are shown below, along with optional markers that may be used by some centres. Core antibodies are required for upload of data; optional markers are not and are listed in alphabetical order. Clones and fluorochromes used should be uploaded for required and optional markers. Where not indicated, clone and fluorochrome choice is dependent on available detectors and filters on the cytometer used at each centre.
Panel A
Type | Antibody (Marker) | Clone | Fluorochrome |
Required | CD5 | 53-7.3 | BV421 |
Required | CD4 | RM4-5 | FITC |
Required | CD44 | IM7 | PE |
Required | CD8a | 53-6.7 | PE-CF594 |
Required | CD25 | PC61 | PE-Cy7 |
Required | CD161 | PK136 | APC |
Required | CD62L | MEL-14 | APC-Cy7 |
Required | Live/Dead | - | SytoxBlue |
Optional | CD3e | 145-2C11 |
|
Optional | CD24 | M1/69 |
|
Optional | CD27 | LG.3A10 |
|
Optional | CD357/GITR | DTA-1 |
|
Optional | CD45 | 30-F11 |
|
Optional | KLRG1 | 2F1 |
|
Optional | Ly6c | AL-21 |
|
Optional | TCRd | GL-3 |
|
Panel B
Type | Antibody (Marker) | Clone | Fluorochrome |
Required | CD5 | 53-7.3 | BV421 |
Required | Ly6G | 1A3 | BV421 |
Required | CD19 | 1D3 | BV510 |
Required | Ly6C | AL-21 | FITC |
Required | CD21/CD35 | 7G6 | PE |
Required | CD11b | M1/70 | PE-CF594 |
Required | CD11c | HL3 | PE-Cy7 |
Required | CD161 | PK136 | APC |
Required | MHCII | M5/114.15.2 | APC-Cy7 or A700 |
Required | Live/Dead | - | SytoxBlue |
Optional | CD23 | B3B4 |
|
Optional | CD27 | LG3.A10 |
|
Optional | CD43 | S7 |
|
Optional | CD44 |
|
|
Optional | CD45 | 30-F11 |
|
Optional | CD317 | 927 |
|
Optional | F4/80 | BM8 |
|
Optional | IgD |
|
|
Optional | KLRG | 2F1 |
|
- Read buffer / dead cell exclusion dye
- SytoxBlue at 1:10000 concentration in FACS buffer OR
- SytoxGreen at 1:20000 concentration in FACS buffer
- Zombie Near Infra-Red live dead from Biolegend at 1:2000 concentration
- Require 200 μl per well (i.e. 400 μl for each spleen).
- Enzyme cocktail (working solution): 3 ml for each spleen, containing final concentrations of:
- DNAse I: 30-100 μg (from 10 mg/ml stock in enzyme buffer stored in single experiment aliquots at -20ºC, do not freeze-thaw stock)
- Collagenase D: 600 Mandl Units (from 30 U/µl stock in enzyme buffer stored in single experiment aliquots at -20ºC, do not freeze-thaw stock)
NOTE: To top up to the 3ml use enzyme buffer; any intermediate dilutions of the enzyme stock solutions should be prepared with enzyme buffer.
Other preparations on the day
- Bring RBC lysis buffer and stop solution to room temperature.
- Prepare wet ice box, label tubes, etc.
Note all centrifuge steps are: 5 min, 400 x g at 8°C
Spleen collection
- Collect the spleen from euthanized mice.
- Remove all fat from the spleen and weigh the organ on a petri dish (do not hydrate the organ before weighing it as this would lead to substantial errors in measurement).
- Place the spleen in a 1.5ml eppendorf tube with 1 mL of sample collection buffer on ice. Use:
- (if spleens are to be processed on the same day) HBSS without calcium, without magnesium but with phenol red OR
- (if analysis will be delayed) RPMI with 2% CS buffer.
Spleen dissociation / digests
If using a GentleMacs tissue dissociator:
- Add the spleen to a GentleMACS C tube containing 3 ml of 1X enzyme cocktail.
- Clip the tube on GentleMACS dissociator and run programme spleen_2.
- Incubate cell suspension for 30 minutes with gentle mixing at least every 5 minutes. Register incubation temperature.
- Run programme spleen_3.
- Add 300 μL of stopping buffer and mix by inversion to block enzymatic digestion and dissociate T cell-dendritic cell interactions.
- Filter cell suspension:
- through 70-80 μm Nylon mesh filter into a 50 mL Falcon tube OR
- directly from C-tubes pour splenocyte suspension through 30 mm CellTrics Partec filters (#04-0042-2316) into 15 ml tubes.
- (optional) Wash the GentleMACS C tube with 5ml FACS buffer, filter and pool with flow-through from previous step.
- Centrifuge for 5 minutes, 400 x g at 8°C and discard supernatant.
- Resuspend total splenocytes in 1 mL cold FACS buffer and keep on ice (this step is not required if counting is performed on the attune).
OR, if performing manual digests:
- Place weighed spleen in 12x75mm tube containing 1ml of collagenase solution in 1X HBSS with Ca2+ and Mg2+ (17-0.2 Wünsch unit/ml)
- Mince into fine pieces using small scissors, place on ice until all samples are minced.
- Add 2ml collagenase (17-0.2 Wünsch unit/ml) to each tube and place in a 37ºC water bath for 30 minutes.
- Tricturate (pipetting vigorously up and down using a 1 mL pipetman) the mixture to break up clumps.
- Spin at 500 x g in a swing bucket rotor for 5 min at 10ºC. Decant the supernatant, rack the tubes or vortex to resuspend the pellet. Add 2ml FACS buffer, mix well by vortexing, take 10 µl for the counting step.
- Dilutions for counting: 2 serial 1:10 dilutions (10µl cells + 90µl FACS buffer, then 10µl of the 1:10 dilution + 90µl buffer.)
- Spin for 5min, 500 x g at 10ºC, decant supernatant, blot the top of the tube, resuspend pellet at 1x108 cells/ml.
Cell counting
- Perform a cell count on an aliquot of the re-suspended cells (adjust concentration according to the cell counter method used).
- Note the cell count, correct for dilution and calculate the concentration in cells per µl.
- Cell count:
- If performed before RBC lysis, pipette the volume containing approximately 4 million cells/well to a 96 well plate in horizontal fashion starting from A1 onwards for panel 1 staining.
- If performed after RBC lysis, pipette the volume containing approximately 1-2 million cells/well to a 96 well plate in horizontal fashion starting from A1 onwards for panel 1 staining.
- Do the same for panel 2 staining in separate wells leaving a few empty rows between the panels to avoid cross contamination.
- Top up to final volume of 100 ml using FACS buffer, centrifuge, discard supernatant and keep plate on wet ice.
Red blood cell lysis, blocking & staining
- Remove plate from ice and add 30 to 100 ml of 1X RBC lysis buffer (at room temperature) to each cell pellet from the previous step.
- Pipette up and down 2-3 times to break up the pellet and ensure complete lysis. Alternatively, vortex the edges of the plates, then pipet quickly once to ensure resuspension is ideal for optimal lysis.
- Incubate for 1 minute at room temperature and then return to ice and add 100 to 200 ml of FACS buffer (to stop lysis) to each well.
Note: Following RBC lysis, every centrifugation step can be performed at 2000rpm for 1 minute in a 96 well plate, which significantly speeds up the protocol. Do take care to resuspend the cells very well to prevent HTS clumping.
- Centrifuge, discard supernatant and resuspend in 200 ml FACS buffer (this step is not required if lysis was performed in 30 µl, since there will be enough volume left in the well for a bigger wash of 200 µl; saves time on a spin).
- Again centrifuge and discard supernatant and resuspend in 50 ml of 1:100 Fc block and incubate on ice for 10 min. Top up to 200 ml using FACS buffer after incubation.
- Take antibody (AB) cocktails from the fridge. In order to eliminate aggregated ABs from your mix before use, centrifuge each AB cocktail for 8 min at 20,000 x g and 4°C. Dilute antibody cocktail to final working concentration with FACS buffer, or Brilliant stain buffer when two or more brilliant violet antibodies are used, to make the AB mix.
- Centrifuge plate, discard supernatant and resuspend in 50 to 100 ml 1X AB mix in appropriate wells for individual panels followed by incubation on ice and in the dark for 20 min.
- If using Sytox Blue/Sytox Green as live/dead discriminator:
- Top up to 200 ml with FACS or Brilliant Stain buffer after incubation. Centrifuge, discard supernatant and resuspend in 200 ml FACS or Brilliant Stain buffer.
- When ready to read plate, centrifuge again and discard supernatant. Resuspend the pellet in 200 ml of read buffer (Sytox Blue diluted 1:10000 in FACS buffer; Sytox Green diluted 1:20000 in FACS buffer).
- If using Zombie NIR dye as live/dead discriminator:
- Add 200 ml of PBS (RT) to all samples
- Spin at 2000 rpm for 1 minute 8°C
- Add 100 ml/well of Zombie Near-IR Live/Dead dye (1/2000) made up in PBS incubate at room temperature for 10 mins, add 200 ml FACS buffer.
General Recommendations for Setting up Cytometer
Set up the analyser to aim acquire 300,000 viable events (live cells) for each of Panels 1 and 2. 500,000 are recommended for panel 2 in order to increase robustness of myeloid population assessment for low frequency populations (macrophages, DCs).
Notes
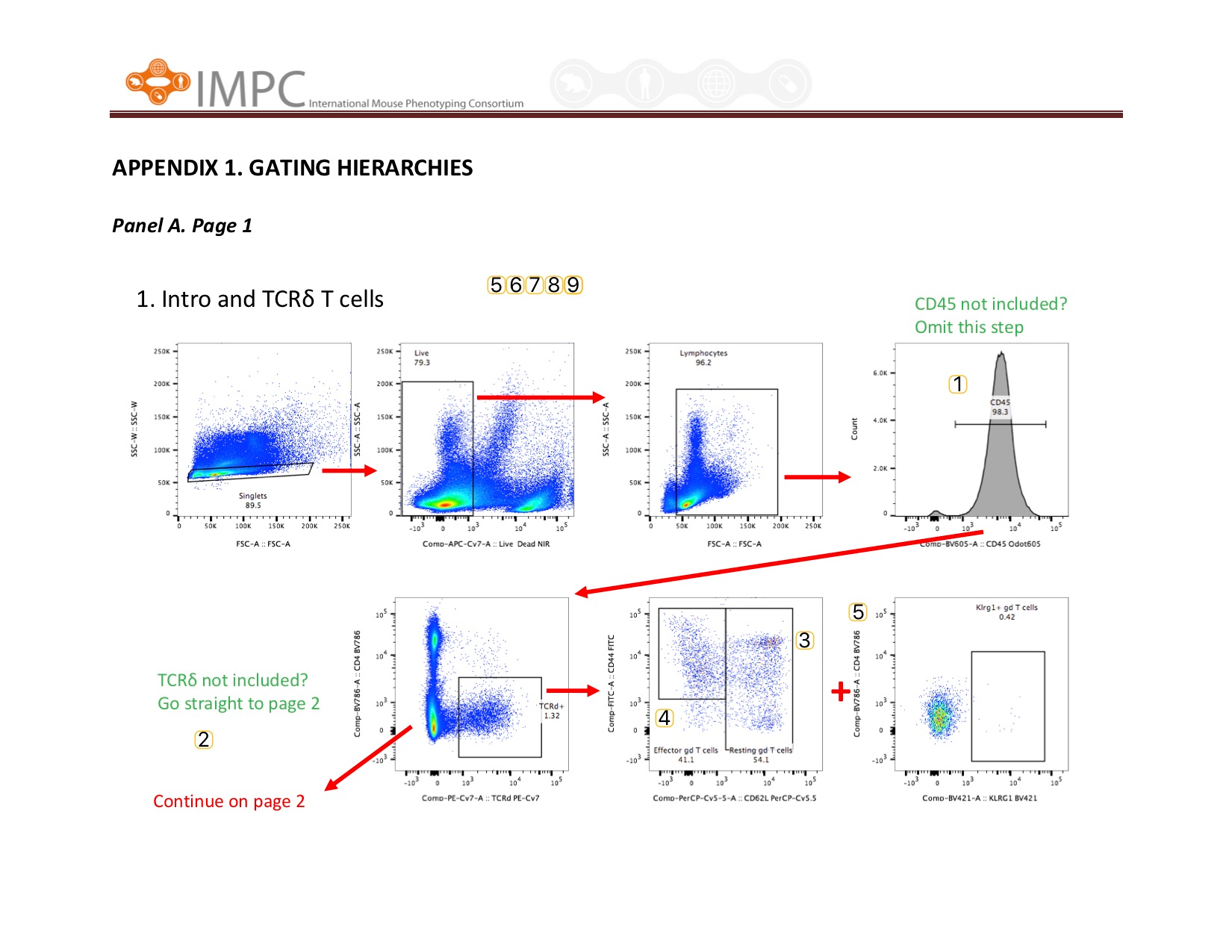
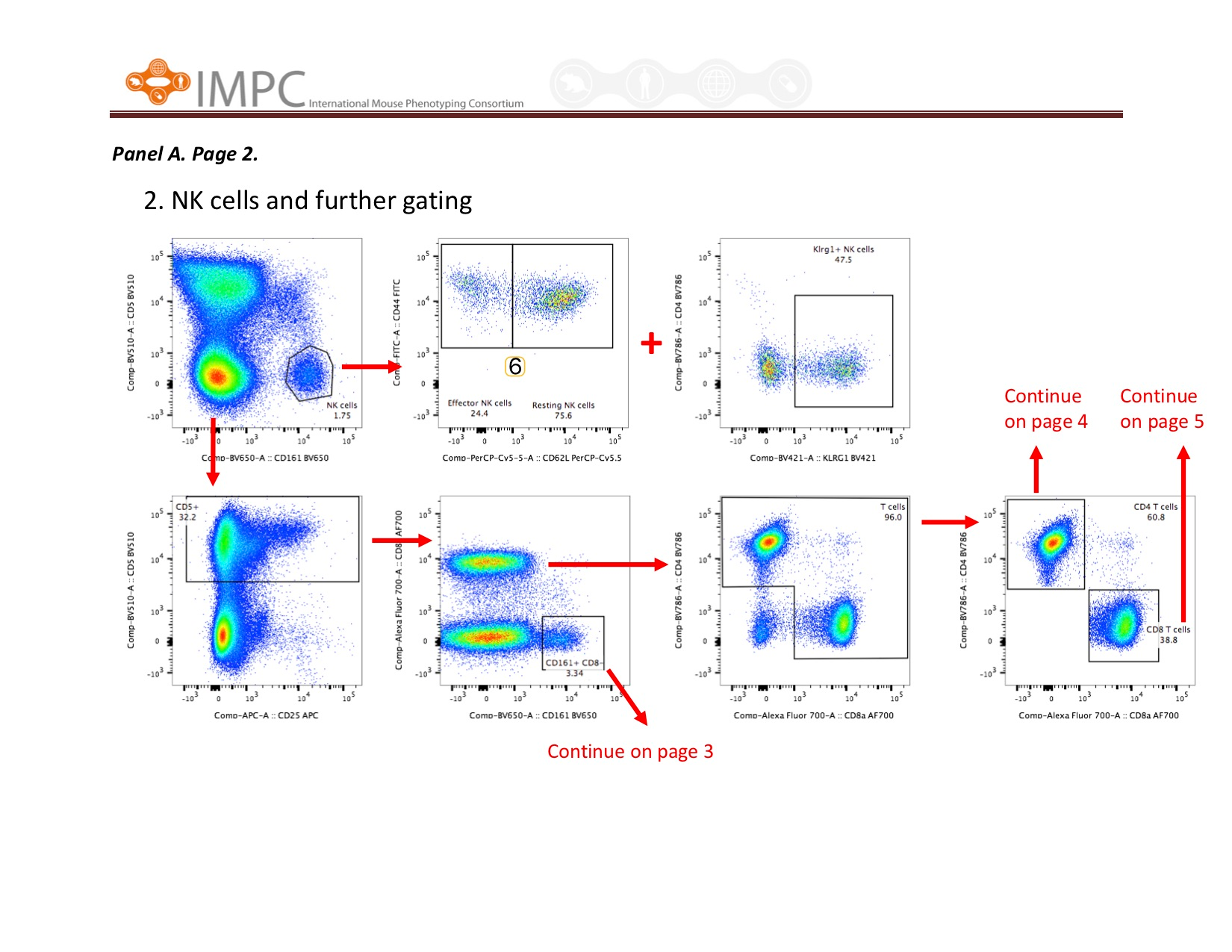
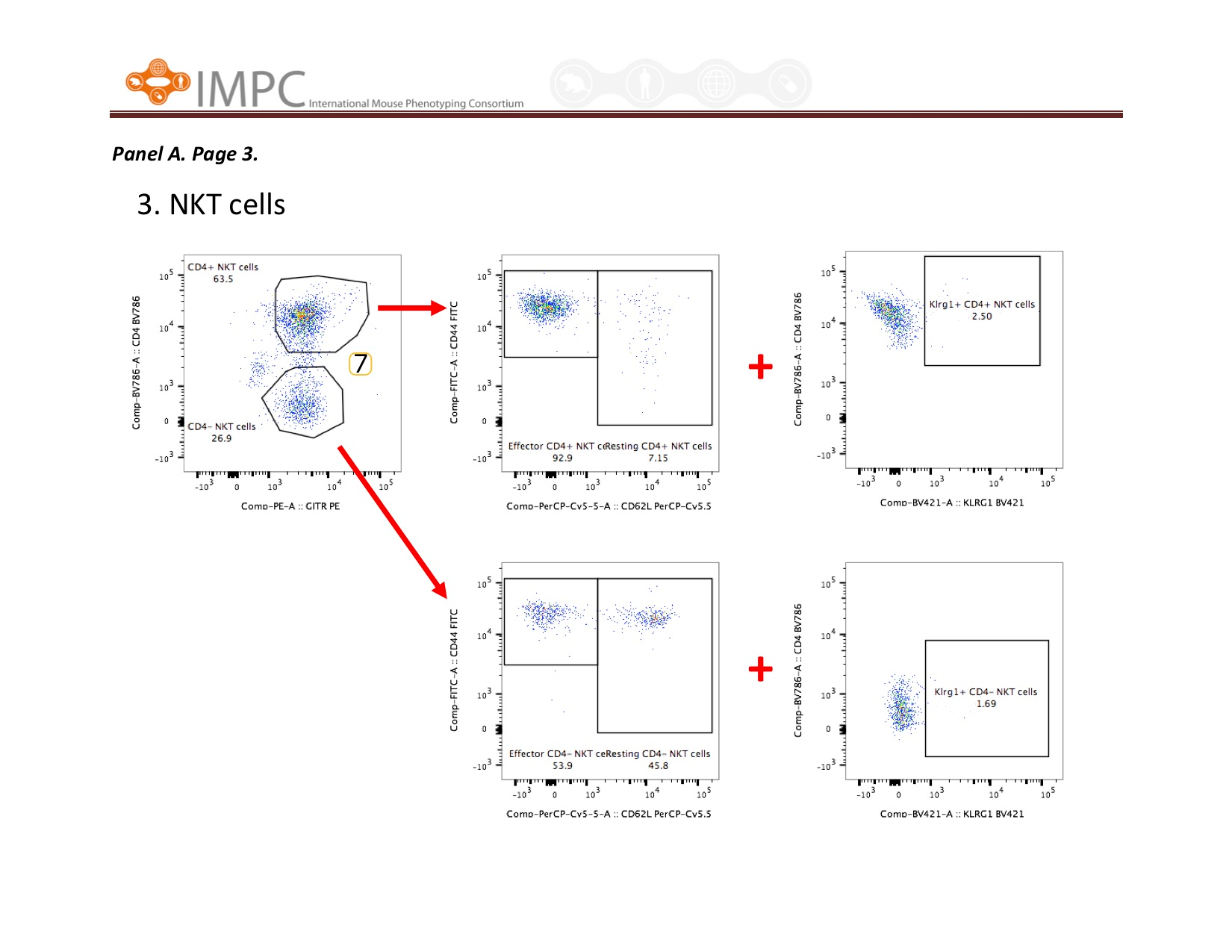
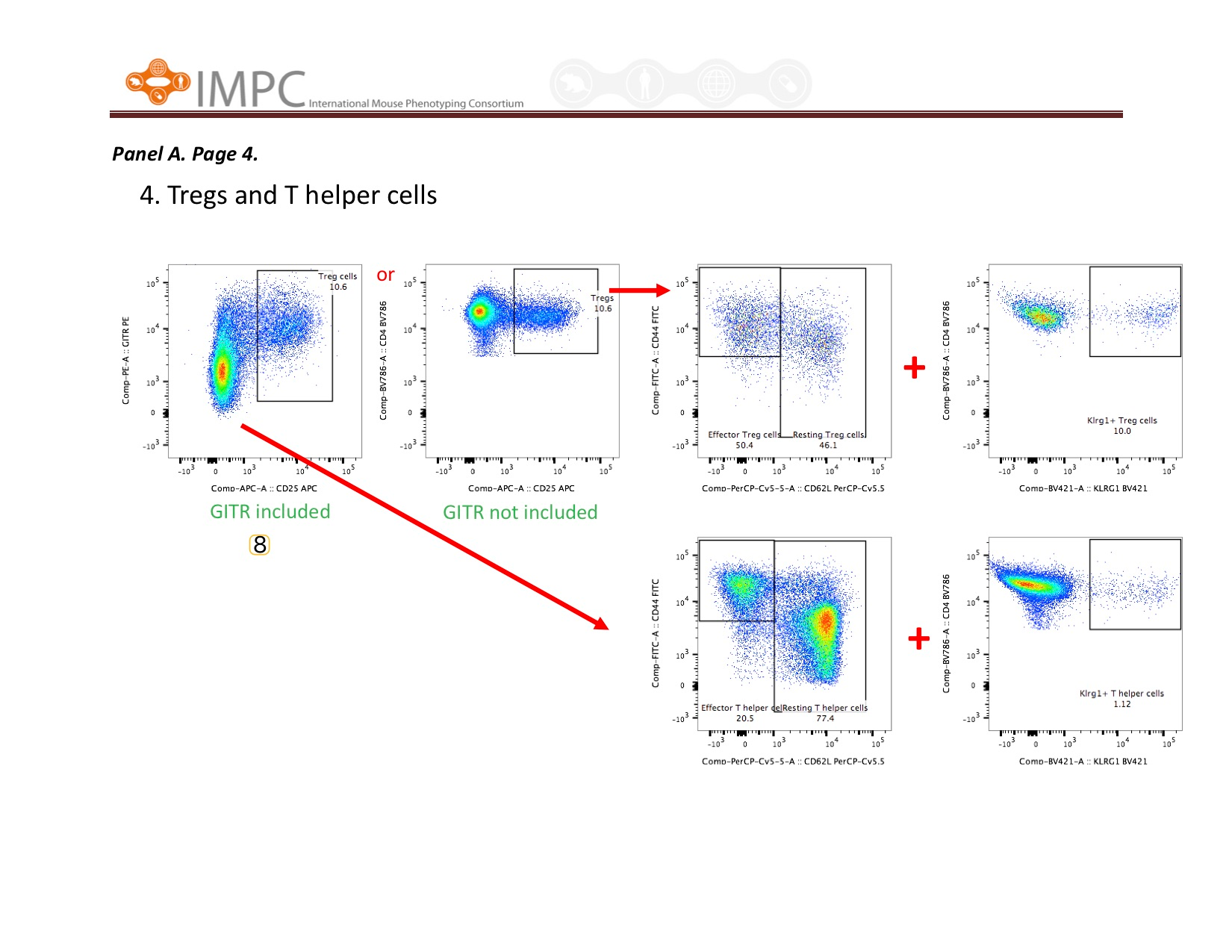
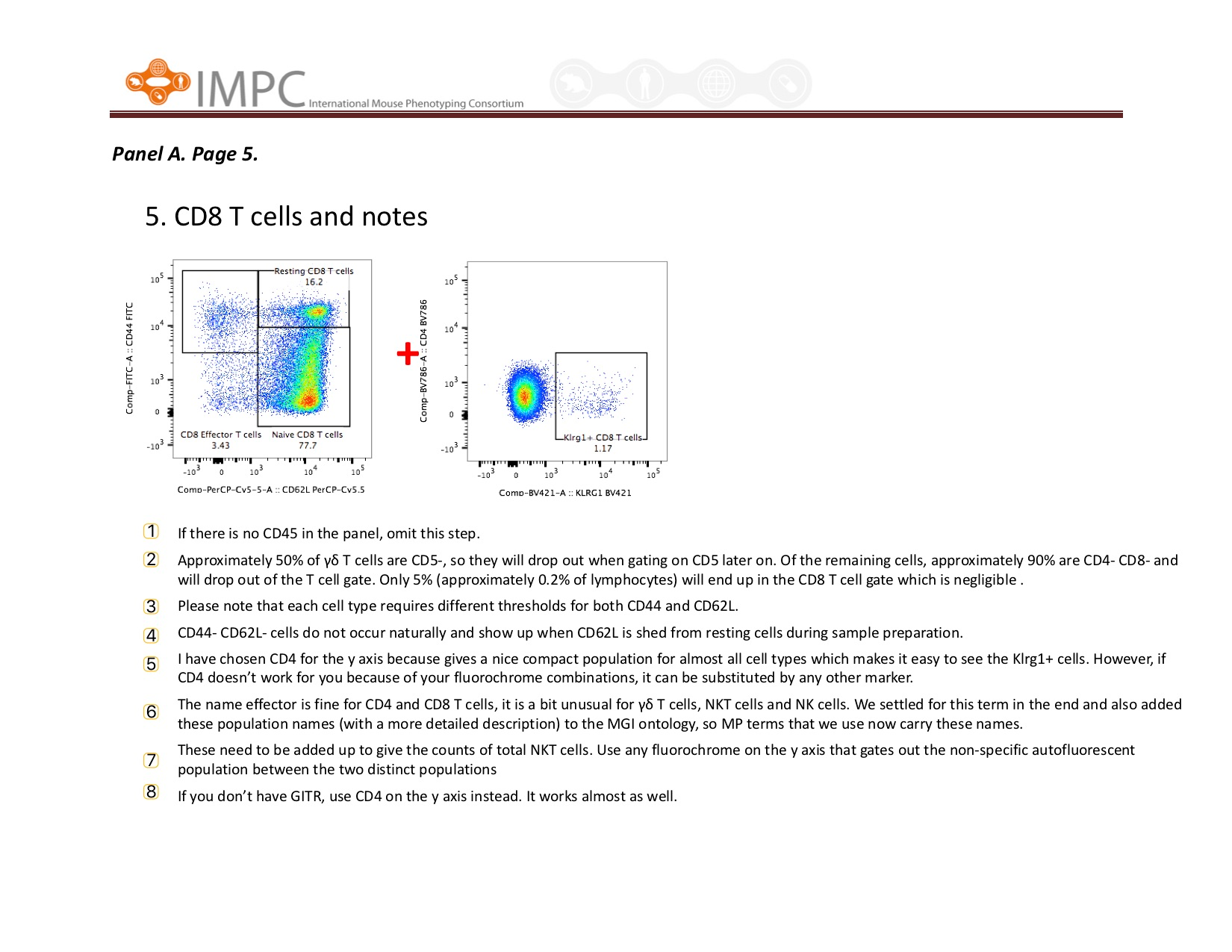
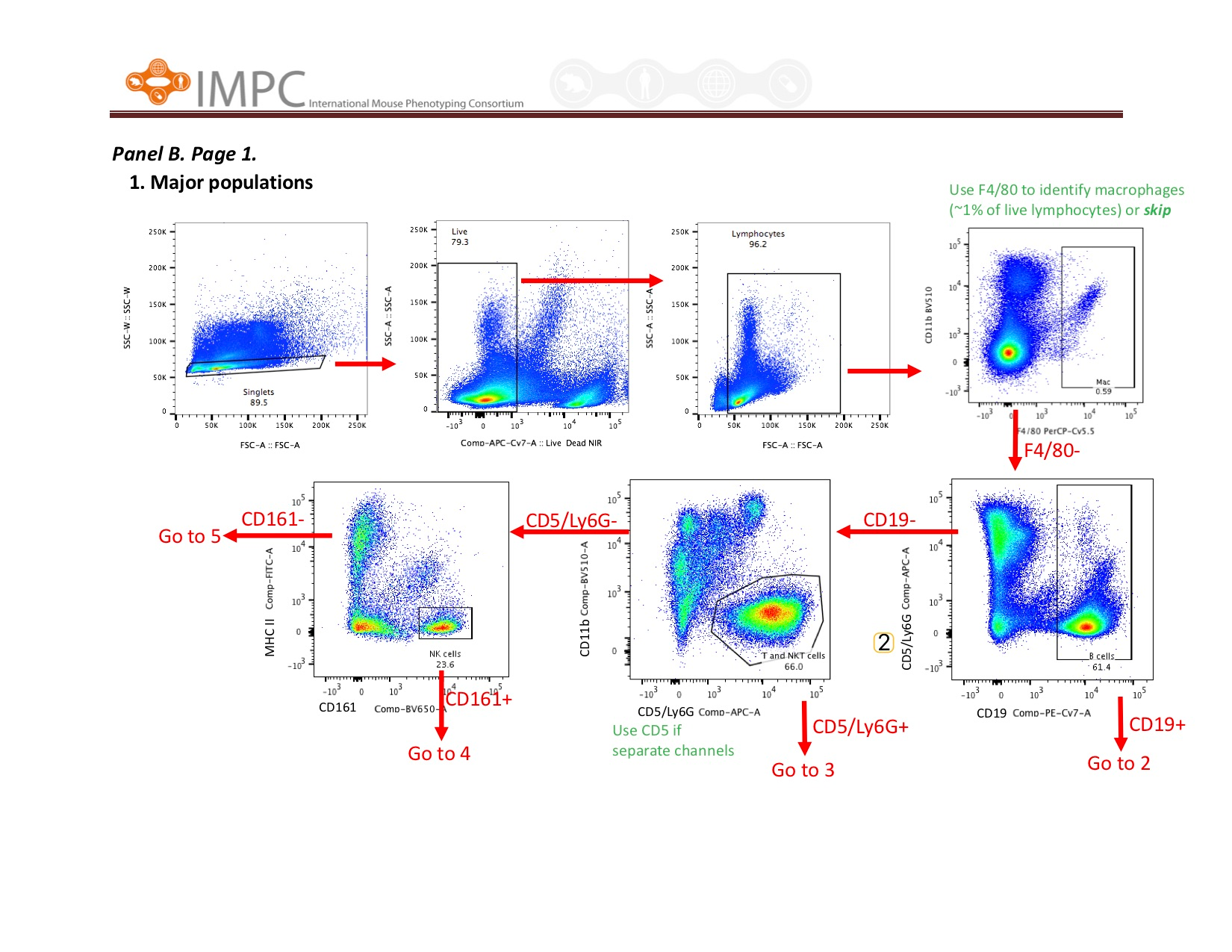
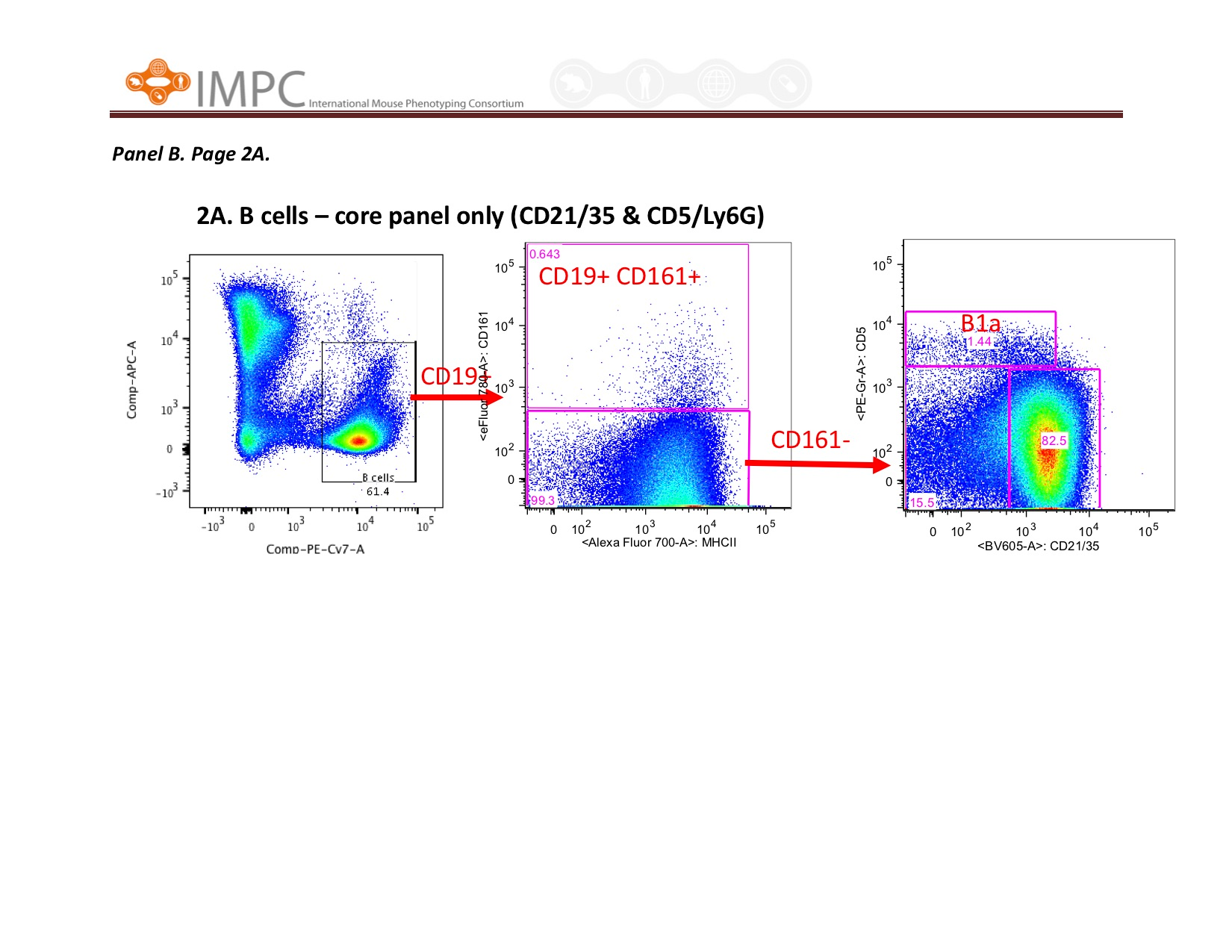
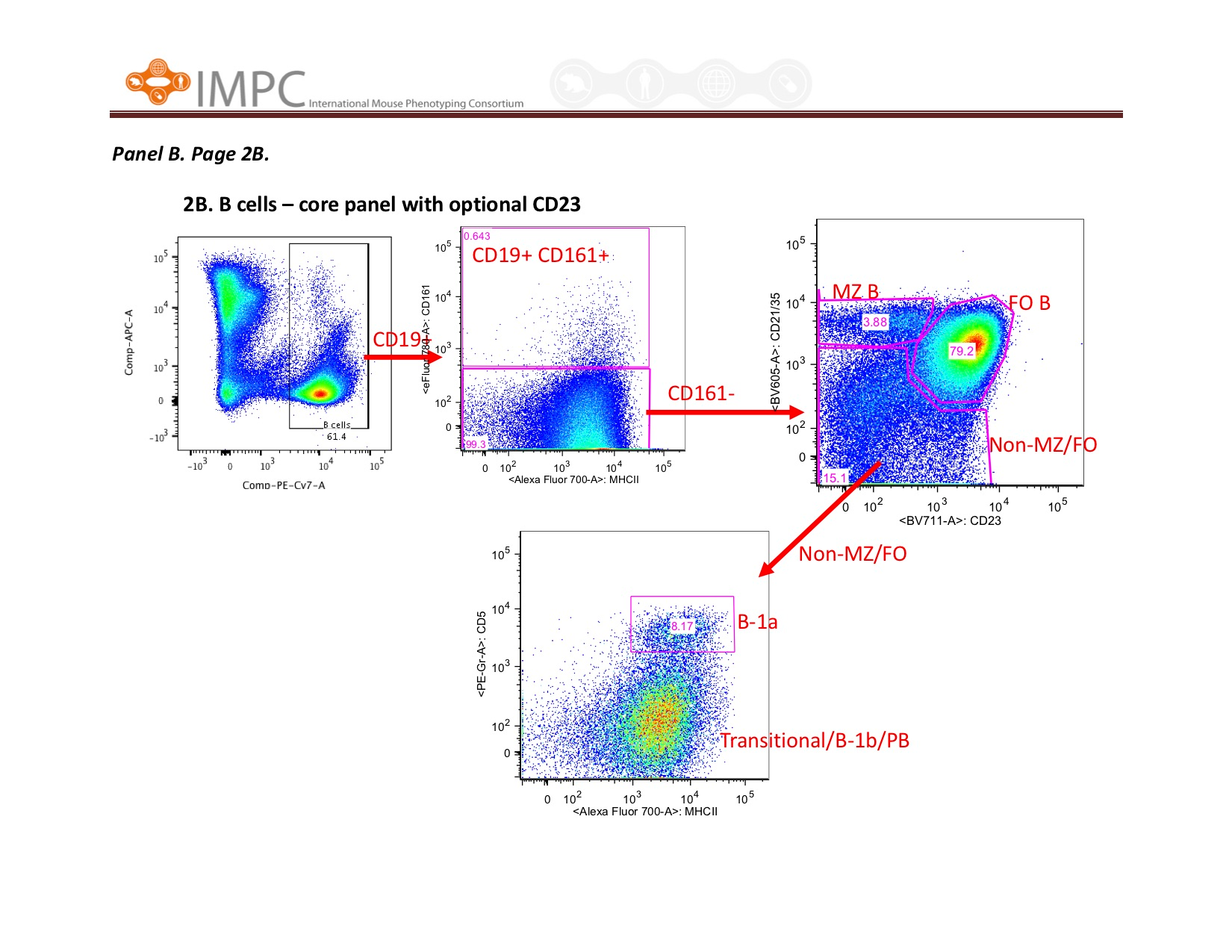
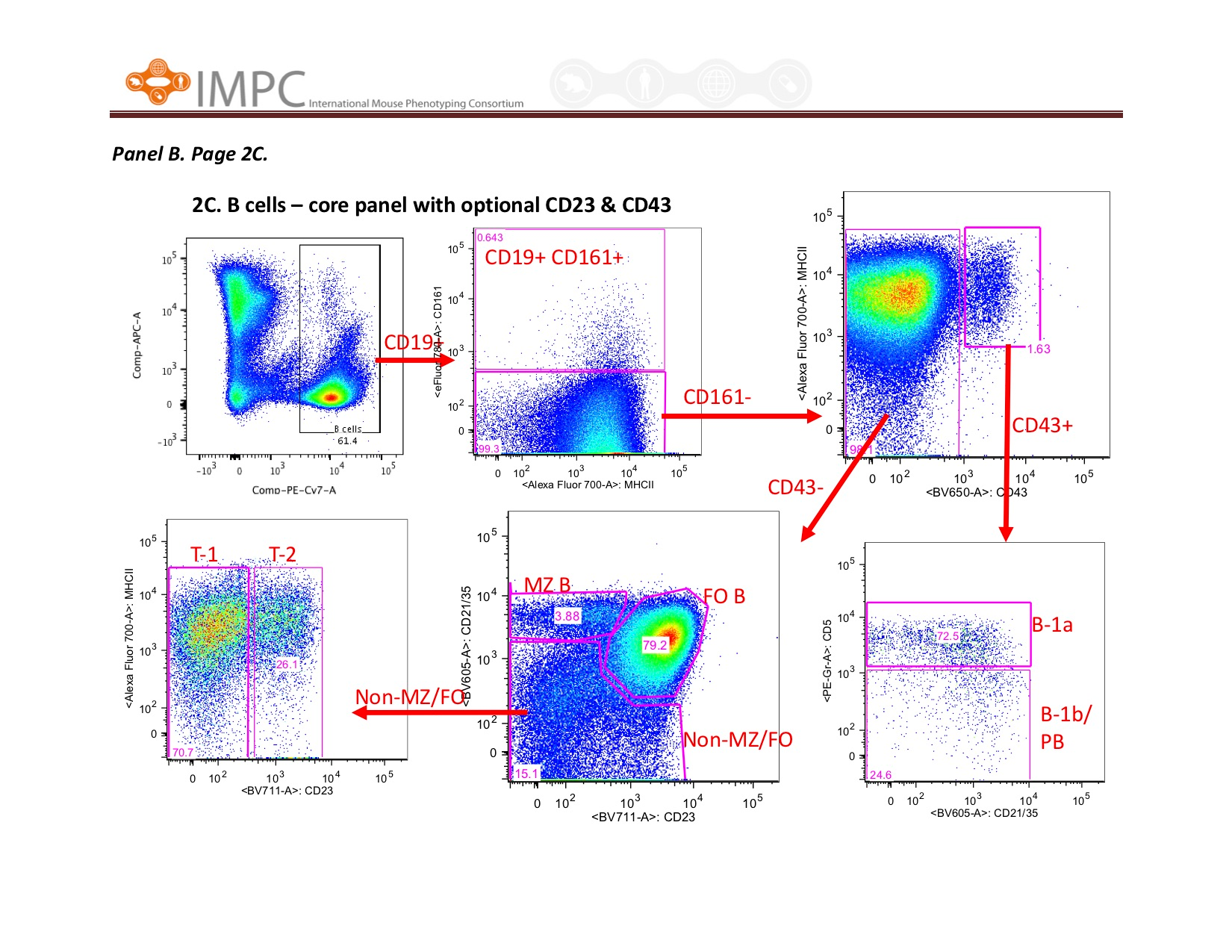
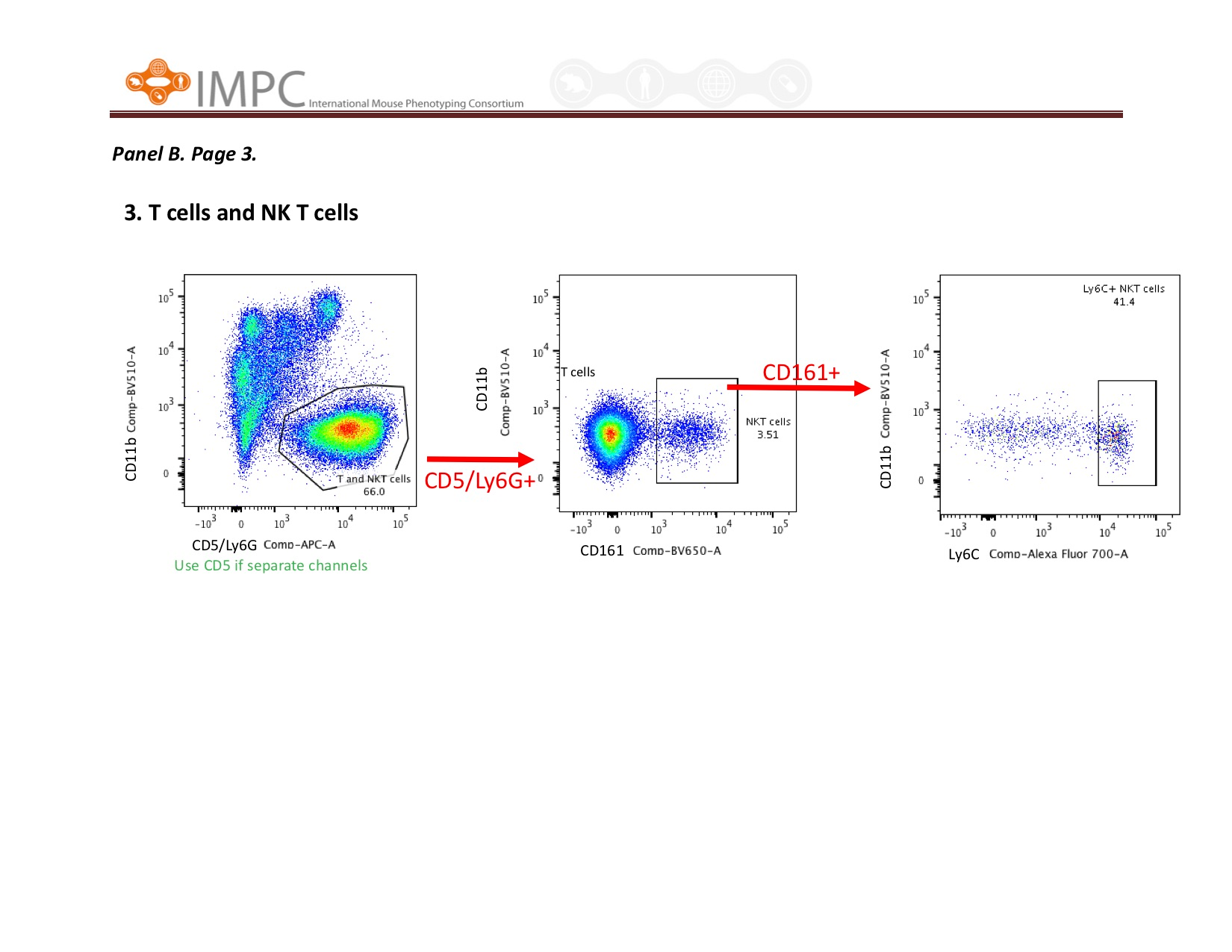
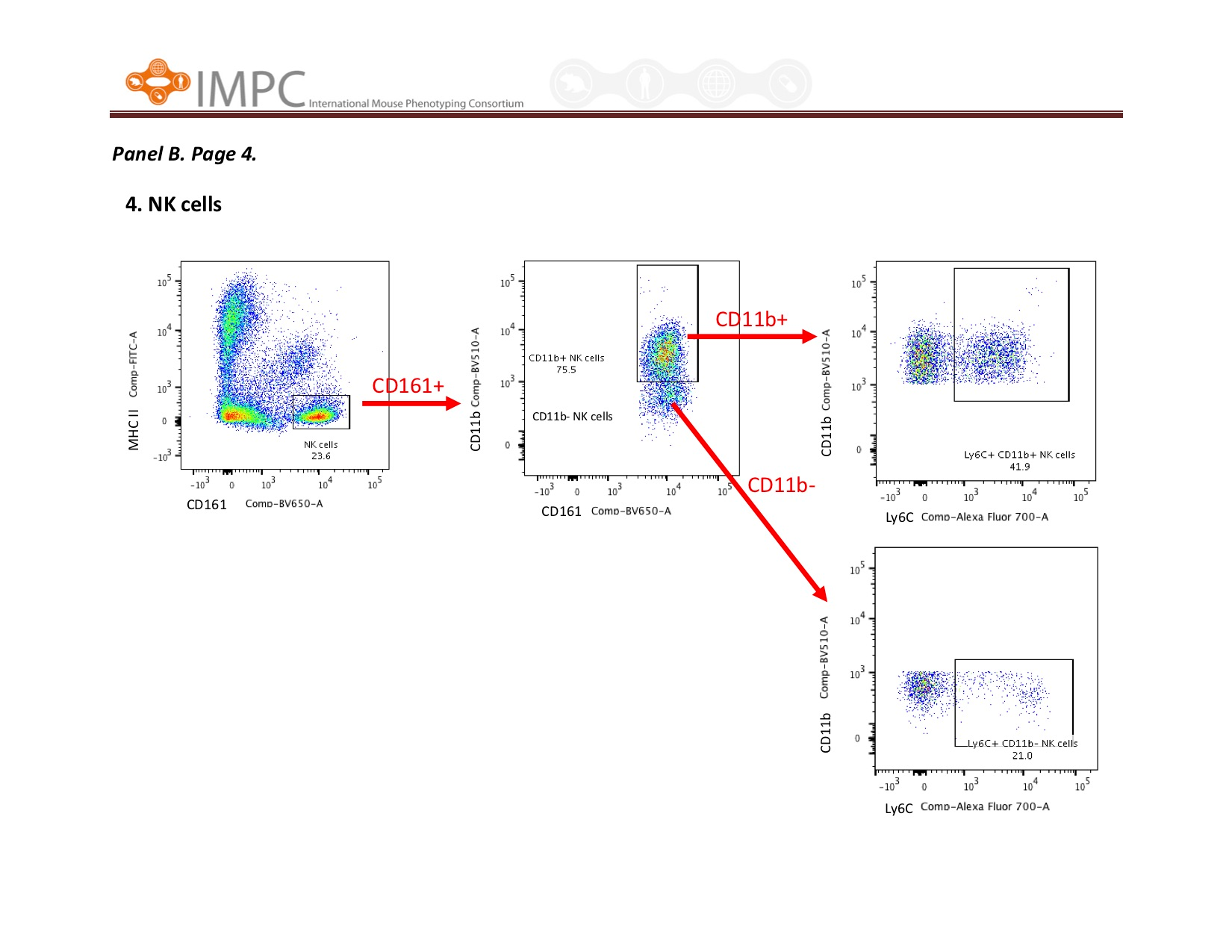
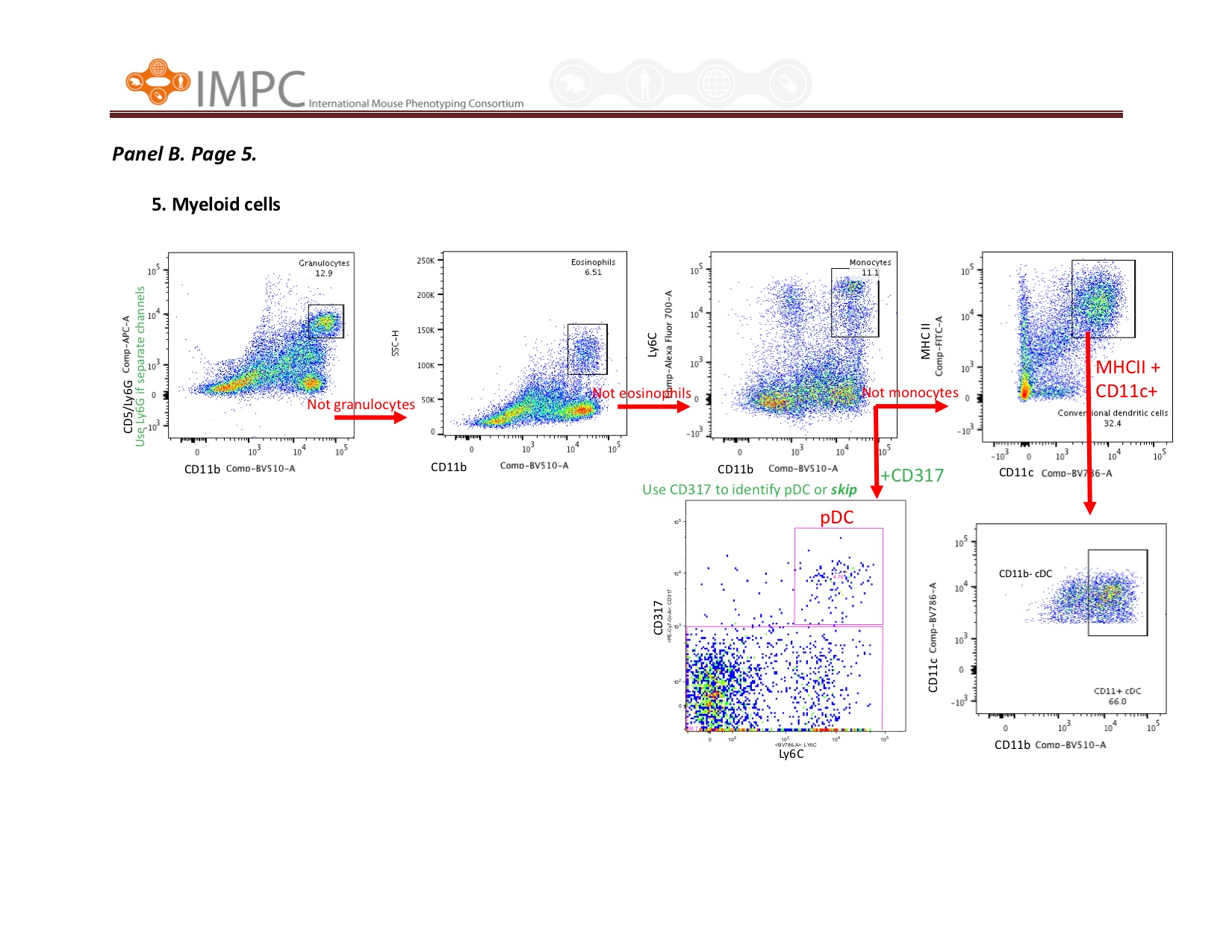
Visual help for Gating