Jump to:
Purpose
Indirect calorimetry provides detailed information on the energy metabolism of mutant mice. Energy expenditure is evaluated through indirect calorimetry by measuring oxygen consumption with an open flow respirometric system. CO2 and O2 sensors measure the difference in CO2 and O2 concentrations in air volumes flowing through control or animal cages. The amount of oxygen consumed over a given period of time can thus be calculated, as far as the air flow through the cage is known. Data are expressed as ml O2 h-1animal-1. The system also monitors CO2 production, therefore, the respiratory exchange ratio (RER) and heat production can be calculated. An activity and food and water intake monitoring system can also be integrated into the set up in order to investigate circadian pattern and behaviour.
Ontological description: MP:0005266 - abnormal metabolism.
Experimental Design
Minimum number of animals : 7M or 7F
Age at test: Week 11
Sexual dimorphism: In general, female mice have higher metabolism compared to males therefore statement is not entirely correct. However, genotype x sex interaction are rare therefore testing only males is acceptable.
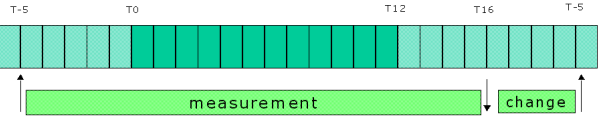
It is essential that all phenotyping experimentation is conducted at the same time of day because physiological and biochemical parameters e.g. metabolic rate, body temperature and activity are subject to temporal rhythms. In the indirect calorimetry module standard measurements begin five hours before lights-off (lights off = T0) and are finished at T16 i.e. four hours after lights-on the next morning. Optional: Mice can be given one day of acclimation before the trial, and the trial can be continued for more than 21 hours.
Equipment
- Calorimetric system equipped with respirometer, feeder and water bottles
- Ambulatory activity monitor (dependent on system specifications)
- Food and water intake monitor
- Computer with apparatus software installed
Procedure
- Optionally mice are allowed to acclimatise to the phenotyping room, to the calorimetry cage, food hoppers and drinking bottles 24 hours before testing.
- Prepare and calibrate the calorimetric apparatus to confirm the accuracy of the gas sensors and flow meters. Specifically prior to each experiment:
- Apply known volumes of CO2 and O2 to determine the sensitivity of the gas sensors and flow meters.
- Run a complete calibration protocol according to the manufacturer’s recommendations.
- Provide each calorimetry cage with sufficient food and water for a period of ~24 (or 48) hours.
- Weigh the mouse.
- Place the mouse into a calorimetry cage with food and water available ad libitum.
- Label the chamber with the corresponding subject identification and close it ensuring there is adequate air flow.
- Initiate the calorimetric system for measurement:
- Set up a new experiment in accordance with the manual (or load a file from a previous experimental setting).
- Start recording measurements five hours before lights off for a total duration of 21 hours at minimum. Optional: 24 hours acclimation can be applied and the recording may continue for 48 hours.
- The latency of CO2 and O2 activity transmitted and recorded is dependent on the number of chambers in use but will be logged periodically.
- Generating a data report:
- Upload all data from the experimentation including:
- Gas analysis VO2 and VCO2 (ml/h/animal)
- Heat production (kJ/h/animal)
- Periodicity of measurements taken throughout experimentation (Figure 1)
- Animal and the corresponding chamber that was used
- The respiratory exchange ratio (RER) can be calculated using the VCO2/VO2 ratio.
- Activity parameters recorded will depend on the specification of calorimetric system used:
- Ambulatory activity can be derived from the number beam splits during the session
- Total activity can be derived from the number of fine movement (e.g. grooming behaviours) as well as ambulatory activity
- An average of each of these parameters of activity is calculated hourly across the measurement period (between T-5 and T16).
- Water and food intake (cumulative, hourly or total food and water intake, between T-5 and T16, will be computable depending on the calorimetric system used).
- Remove each mouse from its chamber in turn at the end of the experimental session and record its weight. Return to their home cage.
- Monitor the animals carefully to observe any abnormal behaviour(s). Ensure that food and water are available ad libitum.
- Wash and wipe clean the chambers with warm water and dilute alcohol or appropriate disinfectant respectively.
Figure 1. Daily workflow of calorimetric experimentation (Note: T0 designates start of dark cycle).
Notes
The system requires periodic calibration of the gas sensors and flow meters to ensure precise measurements. The calibration procedure consists of the application of a gas of known composition and adjusting control knobs in the front of the oxygen and carbon dioxide sensors to obtain readings that reflect the contents of the calibration gas. It is recommended that the system be calibrated prior to the start of each experiment. The analyzers should not be shut down if not urgently required for maintenance. If this has to be done a warm up time of at least 90 minutes is required for the gas sensors for calibration (refer to manufacturer’s manual). Calibrations and shut downs should be recorded in the laboratory journal.
Calorimetry test is to be performed before the ECG/ECHO test to avoid effects of hair removal on the calorimetry results.
The information about the date of the experiment, that is the date when the measurement is performed, is an important parameter which is to be submitted in the Experiment xml file (dateOfExperiment="2013-02-28").
Data QC
- Respiratory Exchange Rate (RER) is between 0.7-1.00
- Mice show normal feeding and drinking behaviour
- Mice show stable weight before and after calorimetry
- Correct calibration of gases according to manufacturer’s manual
MetaData and examples
|
Metadata |
Example |
|
Time of dark cycle start |
The starting time of the dark cycle. E.g. 7 pm |
| Time of dark cycle end |
The ending time of the dark cycle. E.g. 7 am |
|
Room temperature |
The range of min. and max. temperatures of the calorimetry chamber. If the calorimetry machine doesn't register the chamber temperature, the room temperature in which the machine resides can be reported instead. E.g. 20.0-24.0 (C°). If the temperature is constant throughout the experiment, a single temperature can be submitted. E.g. 20.0 (C°). Do not submit the subtraction between the 2 values! |
|
Acclimation to calorimetry cages |
E.g. Yes/No. |
|
Duration of test |
Duration of the test without including the acclimation period. Can be a minimum of 21 hours or more, when acclimation is done. E.g. 21 (hours). |
|
Equipment ID |
ID of the machine used when more than 1 is used having same model and manufacturer. E.g. machine 1, machine 2, machine Minnie, machine Mickey Mouse, etc. |
|
Equipment manufacturer |
Manufacturer of the equipment. E.g. TSE Systems GmbH. |
|
Equipment model |
Model of the equipment. E.g. Labmaster CaloSys. |
|
Experimenter ID |
An ID of any format to be used coherently both inside the same procedure and for all procedures. E.g. Harw_001, or 1/2/3. |
|
Date equipment last calibrated |
Most recent date in which the equipment (or any part of) used in the procedure was subject to a calibration event. |
|
Outer dimension of cage |
(L, cm) x (W, cm) x (H, cm). E.g. 21.4 x 11.5 x 13.3 |
|
Inner dimension of cage |
(L, cm) x (W, cm) x (H, cm). E. g. 21.4 x 11.5 x 13.3 |
|
Height from platform** to lid assembly |
cm. E.g. 8.6 |
|
Available space for mouse |
(L, cm) x (W, cm) x (H, cm). E.g. 20.3 x 10.4 x 8.6 |
| Infrared beam setting on X axis | Orizontal activity: locomotion. N beams with L cm spacing, H cm above platform**. E.g. 16, 1.3, 3.0 |
| Infrared beam setting on Y axis | Orizontal activity: locomotion. N beams with L cm spacing, H cm above platform**. E.g. 16, 1.3, 3.0 |
| Infrared beam setting on Z* axis | Vertical activity: rearing/jumping. N beams with L cm spacing, H cm above platform**. E.g. 16, 1.3, 6.8 |
|
Beam strip placement on exterior of chamber**** |
Left/right, front/back, both |
|
Lightbeam wavelength |
nm, E.g. 950 |
|
Scanning rate |
Hz, E.g. 100 |
|
Refractory period |
s, E.g. 0.8 |
|
Presence of bedding into the cage |
Yes/No |
| Igloo in cage | Yes/No |
*Z has same direction as X only is higher with respect to the platform
** the floor where the mouse stand
*** This value is the "Height from platform** to lid assembly"